8 Often Overlooked Recruitment Roadblocks that Hold Trials Back and How to Overcome Them
- FutureMeds

- Dec 8, 2022
- 5 min read
Updated: Jan 24, 2023
About 50% of sites selected for clinical trials endanger trial timelines due to poor patient recruitment performance. Therefore, recruitment teams and patient engagement teams are recommended to address underlying recruitment issues so we, as an industry, can move forward faster together.

Patient recruitment remains one of clinical development's most significant and costly bottlenecks. It is one of the top 3 issues that keep sponsors and CROs awake at night. And for a good reason: four out of five clinical trials experience delays, costing between $600,000 - $8 million daily, wasting precious time, burning participant confidence and compounding problems.
With the trend toward more targeted patient populations and more complex clinical trial design, traditional recruitment practices may require retooling and additional strategies and tactics need to be devised and deployed to meet the demand for qualified participants.
According to research from the Tufts Center for the Study of Drug Development (CSDD), 11% of sites selected for clinical trial studies fail to enrol even a single subject and 37% under-enrol. Although 89% of studies eventually meet enrollment goals, hitting targets often comes at the cost of doubling the original timeline.
So the question remains, in a complex, ever-changing clinical research environment, how can research sites, Sponsors and CROs identify and tackle the right problems to mitigate delays?
Over the course of more than 80 semi-structured interviews with investigators, who have personal experience with discontinued trials, and other stakeholders, a recent study, “Exploring reasons for recruitment failure in clinical trials,” identified 29 different reasons for recruitment failure.
“Overoptimistic recruitment estimates, too narrow eligibility criteria, lack of engagement of recruiters/trial team, lack of competence/training/experience of recruiters, insufficient initial funding, and high burden for trial participants were mentioned most frequently.”
The researchers also unearthed 8 new previously unpublished reasons for recruitment failure:
Patients not seeking trials in high-quality healthcare systems with many options (lack of incentive)
Decentralised healthcare systems with many small hospitals resulting in a limited number of referrals
Insufficiently compelling therapeutic areas or research questions (e.g., perceived as little relevant for patients)
Too few recruiting sites planned or too few study staff (e.g., recruiters, limited engagement of study nurses)
Lack of patient engagement in trial design/planning
Lack of competence/training/experience (includes inadequate planning; enthusiasm is not enough,” staff turnover)
“Trial fatigue” (motivation compromised due to recurrent prolongation of recruitment period)
Lack of trust due to a short-term relationship with the healthcare team (e.g., acute care vs chronic conditions (e.g., dialysis patients)
Although this is the first paper that published these insights, most research sites across the globe are somewhat familiar with the problem. Compared to traditional trial settings where other non-trial-related burdens and challenges can interfere with trials, organisations that focus on clinical research, such as FutureMeds, are more likely to have working solutions to the challenge. A simple reason, they have to; without healthy recruitment numbers, they can’t stay afloat and can't stay on mission.
At FutureMeds, our Dedicated Research Site and Patient Engagement teams are acutely aware of these recruitment challenges and the specific problem areas that can cause severe delays. Our teams’ proactive recruitment and retention approach and our comprehensive understanding of country-specific limitations, advantages and patient populations and journeys combined with DCT elements may provide winning advantages in easing patient recruitment challenges and increasing the flexibility of trial conduct.
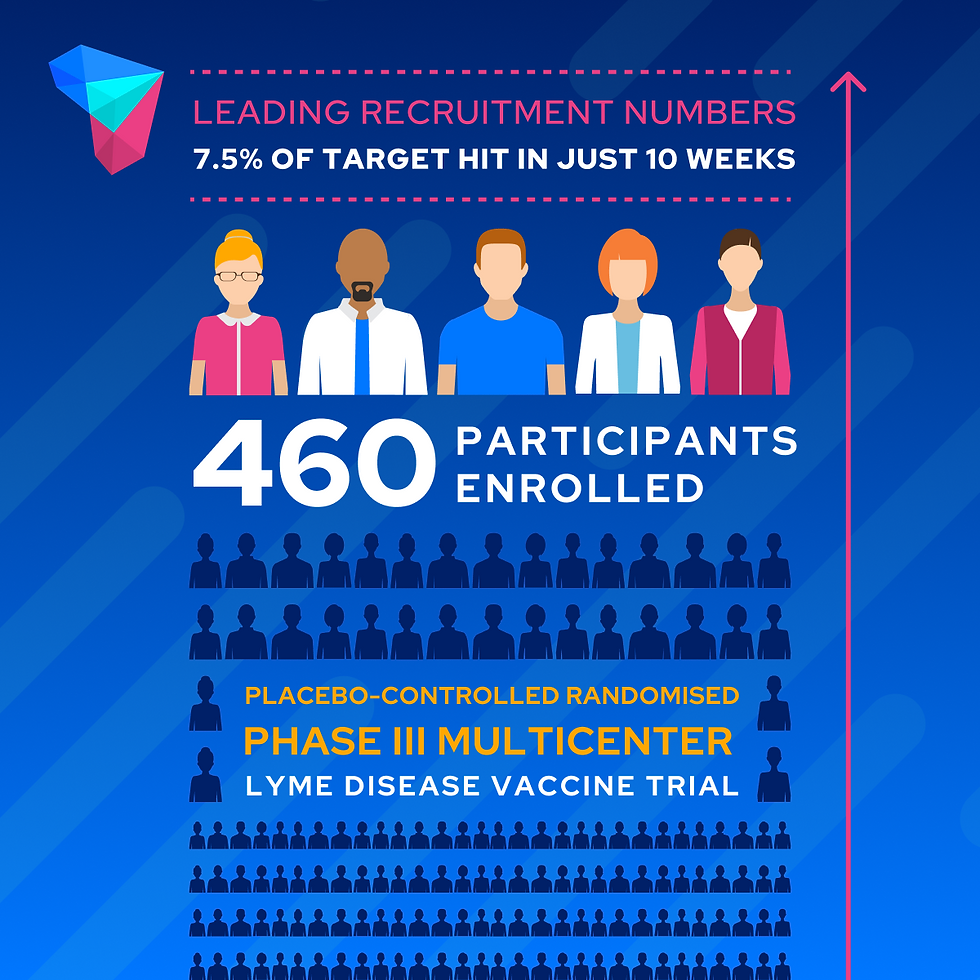
Recent enrolment figures of only two FutureMeds Dedicated Research Sites (enrolled 7.5% of the global target population in 10 weeks) show that the FutureMeds model accelerates patient recruitment and, as a result, can mitigate study delays.
FutureMeds 3 key pillars for effective patient recruitment
Referral relationships built on trust that widens patient choices
We are on a mission to forge strong and lasting ties with healthcare providers across various therapeutic areas to help them provide patients with the best choices and treatment options.
FutureMeds’ Clinical Relations team forge lasting ties with professionals and healthcare providers across primary and secondary care providers. Last year, existing and new Polish referral partners helped our teams deliver more than 300 randomizations. These referral partners also introduced a few thousand patients to FutureMeds and highlighted the benefits of clinical trials. All of these patients are potential candidates for future clinical trials.
To put the above numbers in perspective, FutureMeds Poland’s CRS team currently delivers approximately 35% of site targets, making them the most effective patient source in the country.
A fast-growing patient database that boosts patient access to treatments
Marketing, recruitment and clinical relations activities go hand in hand with database management. A well-designed and well-maintained database system not only underscores clinical trial operations and protects research timelines but also helps patients access better care.
Collaborations across teams that increase patient experience
Looking at the data across our studies, we found that optimising retention is one of the most effective ways to minimise risk to study timelines. It all starts with fully understanding the patient. Once we have a better understanding, recruitment of patients becomes a lesser challenge.
“We aim to establish FutureMeds as the first choice for patients looking for new therapeutic options. To make this happen, we are building and optimising our technological and marketing stack whilst implementing strategies that help our teams grow clinical trial awareness among patients and healthcare providers in Europe.”
- Arkadiusz Kasperski, Head of Patient Engagement at FutureMeds
Why choose FutureMeds
At FutureMeds, we believe everyone, everywhere, deserves a better future. It all starts with building trust in clinical research, in our service and strengthening professional relationships with the medical community.
On average, our PIs have 15 years of clinical trial experience, are well-established in their communities, and are all driven to help progress medical science and provide better care for patients across the globe.
Recruiting quality investigators and investigative sites directly correlates to quick participant enrolment, retention and quality data for any clinical study. By addressing the underlying recruitment and retention challenges, the FutureMeds Dedicated Research Site model offers Sponsors and CROs significant efficiencies and savings across study timelines.
About FutureMeds
FutureMeds is a fast-growing independent Dedicated Research Site Network supporting pharmaceutical companies, Sponsors and CROs and contributing to research to find effective treatments and medications for all patients who need them.
FutureMeds’ Dedicated Research Site teams strive to accelerate study timelines, streamline processes, lower costs and improve data quality to help accelerate patient access to new treatments.
Through acquisitions and a strategic focus on patient experience, FutureMeds has developed qualified patient pools across Europe that enables faster patient enrollment and strong retention and help generate more accurate results.
Follow us on LinkedIn
Follow FutureMeds on LinkedIn and join a growing group of engaged, passionate healthcare and pharmaceutical professionals who are on a mission to accelerate the drug development process and achieve regulatory approval faster so patients can safely access the very latest treatments as soon as possible.


Comments